Argus
A Dravet Syndrome
Clinical Trial
The Argus Trial is a multicenter clinical study investigating
Clemizole in children and adult participants with Dravet syndrome.

The Argus Trial is also looking at:
- If there are any safety concerns for participants.
- How the study drug is processed by the body.
Up to 150 participants with Dravet syndrome will take part in this global research study.
About The Argus Trial
The Argus Trial is being conducted to see if the investigational medicine, Clemizole, lowers the number of seizures in participants with Dravet syndrome who are 2 years of age and older. Although this is the first time that Clemizole is being studied in people with Dravet syndrome, the safety, tolerability, and the way the body processes the investigational medication have previously been studied in a small number of healthy adults.
Study participants will be carefully monitored for safety during the clinical trial. Participants have a 1 in 2 (50%) chance of receiving either the investigational medication, Clemizole, or a placebo (a liquid that looks like the investigational medication but contains no active medication). Neither you nor the study staff will know which one the participant is receiving. Participants who complete the first 4 months of the clinical trial may have the opportunity to receive Clemizole in the optional extension period at no cost to them. Participants must remain on the antiseizure medications they are currently taking during this clinical trial.

Why Participate
If a person living with Dravet syndrome qualifies and decides to participate, they will receive:
- All study-related care and the investigational medication at no charge
- Assistance with study-related travel expenses (including food and lodging), as needed
- Close monitoring by a study doctor and study team
- The opportunity to help researchers learn more about Dravet syndrome
Safety while participating in the Argus Trial is our highest priority. If any questions or concerns arise, a study team member is available to help.
Participation in any research study is always voluntary. Participants are free to leave a clinical trial at any time and for any reason. Privacy will be maintained throughout the Argus Trial.
Download the Argus Trial Brochure
The Argus Trial brochure provides information similar to this website to help you discuss participation in this research study with the potential participant's doctor. You can also share it with someone who may want to learn about the Argus Trial.
Get the Argus Trial BrochureAbout Dravet Syndrome
Dravet syndrome is one of the most drug-resistant forms of epilepsy. Eighty percent (80%) of cases of Dravet syndrome are caused by a genetic mutation. However, the cause in other cases remains unknown. The disorder usually appears during the first year and not only results in persistent drug-resistant seizures but also causes intellectual and developmental delays. Additionally, children with DS typically experience hyperactivity, sleep difficulties, growth and balance issues, and difficulty relating to others.
Argus Trial Quick Facts
The Argus Trial is a multicenter, multinational study evaluating an investigational medication, Clemizole, to see if it may reduce the number of Dravet syndrome seizures in children and adults. This study includes 4 parts: Screening, Double-Blind Period, Long-term Extension, and Follow-up. Participants who complete the first 4 months of the study may have the opportunity to receive the investigational medication (Clemizole) for an additional 3 years. The study doctors will share the details with you.

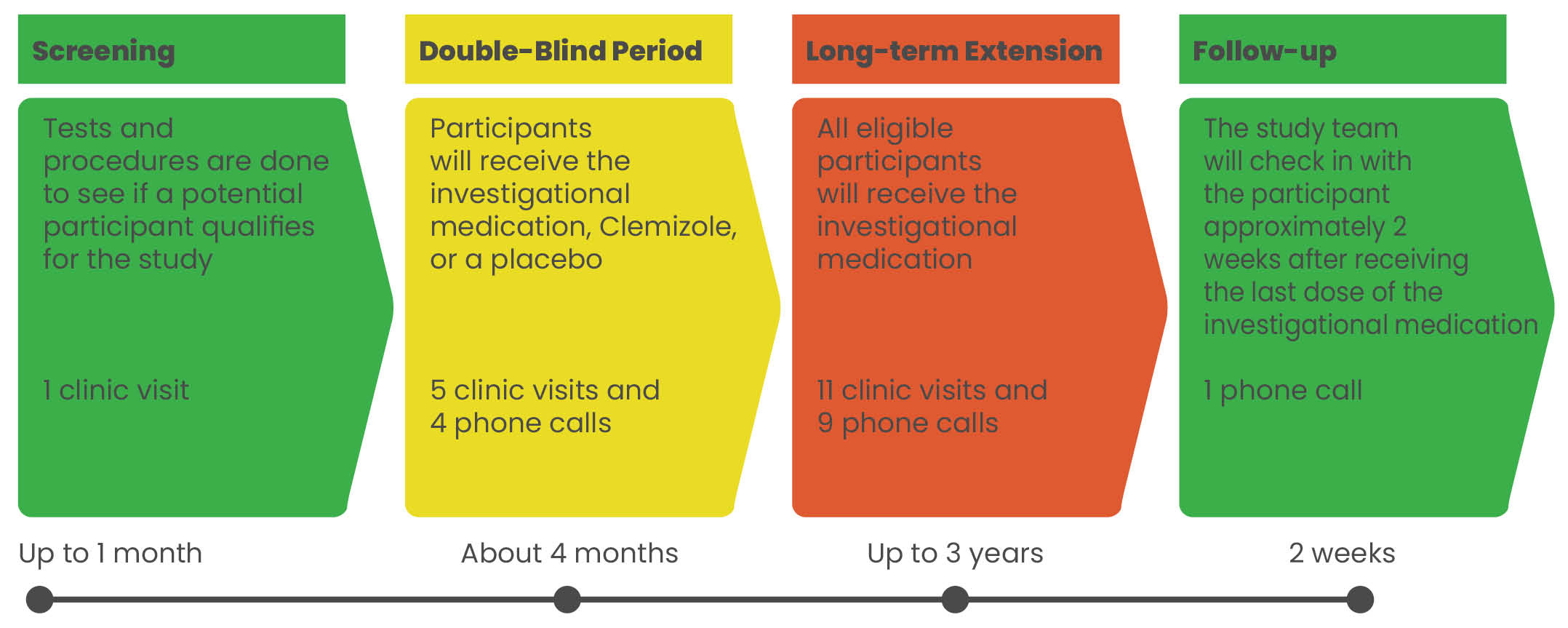
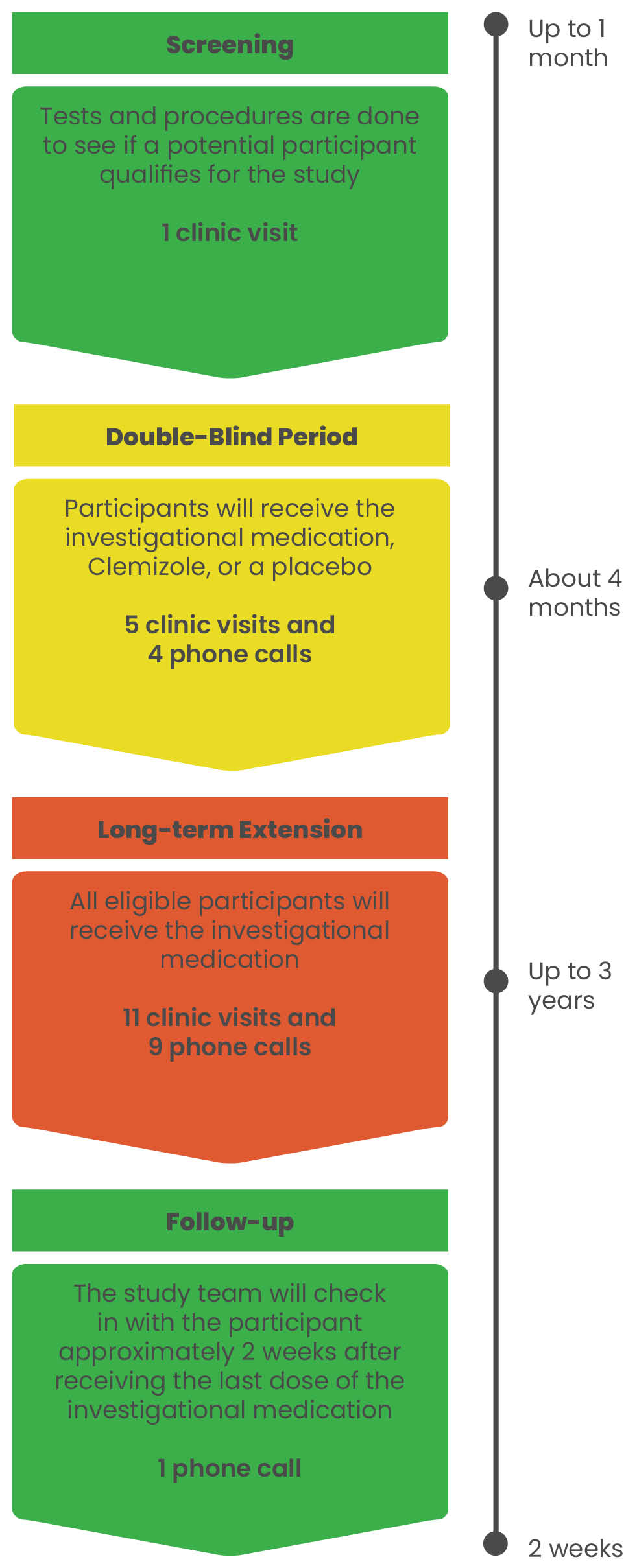

Study Drug
EPX-100, clemizole hydrochloride, is an investigational drug product which is being evaluated for the treatment of seizures associated with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS). These are serious conditions that affect the brain and cause seizures. The exact way EPX-100 affects seizures in people with epilepsy is being studied, but it is thought that it likely works by interacting with serotonin receptors. Serotonin is a chemical found in the body which is thought to be involved in mood, sleep, appetite, and other bodily functions. EPX-100 is administered orally twice a day in a liquid formulation.
Seizure and medication diary: You will fill out a seizure and medication diary at home to keep track of the participant’s seizures and when they take the investigational medication. The seizure diary is important to help researchers understand the effects of the investigational medication on the participant’s seizures.
How to Qualify
People living with Dravet syndrome may be able to participate in the Argus Trial if they*:
- Are at least 2 years old
- Have a genetically confirmed diagnosis of Dravet syndrome
- Experienced onset of seizures prior to 18 months of age.
- Lack seizure control despite taking 1 or more antiseizure medications.
- Have frequent seizures (4 seizures per month within last 28 days)
- Are not enrolled in another clinical study
*This is not a complete list of study requirements. The study doctor will review all the requirements with you and/or the potential participant.
Interested Parents and Caregivers
Study-related care and the investigational treatment will be provided to eligible study participants at no cost. In addition, travel for study-related visits will be provided for study participants and a caregiver, if applicable.
Study Locations
To find a clinical site near you, select a region below the
search box.
Please contact the study coordinator at the
nearest site listed or find contact information at https://clinicaltrials.gov/ct2/show/NCT04462770
British Columbia Children's Hospital
4480 Oak Street Room K3-167 Vancouver, British Columbia V6H 3V4
-

Mary Connolly
-

604-875-2121
University Health Network
Toronto Western Hospital 5W-445, 399 Bathurst Street Toronto, Ontario M5T 2S8
-

Danielle Andrade
-

416-603-5800 ext. 5906
Children's Hospital of Eastern Ontario Research Institute Inc.
401 Smyth Road Ottawa, Ontario K1H 8L1
-

Katherine Muir
-

613-737-7600 ext. 1605
The Hospital for Sick Children
555 University Ave Toronto, Ontario M5G 1X8
-

Elizabeth Donner
-

416-813-7996
Hospital Infantil Universitario Niño Jesús
Avda de Menendez Pelayo, 65 Madrid, Espana, 28009
-

Victor Soto
-

+34 660 56 58 97
Hospital de la Santa Creu i Sant Pau
C/ Sant Antoni Maria Claret, 167 Barcelona-08025
-

Alba Sierra
-

+34 633 16 08 18
Medical Centre Plejady
Szafrana 5D – Fi estate Krakow, Poland 30-363
-

Marta Zolnowska
-

+48 796 975 720
Institute of Mother and Child
Kasprzaka 17 A street, Warsaw, Poland 01-211
-

Elżbieta Stawicka
-

+48 604 448 043
University of Debrecen
University of Debrecen Pediatrics Clinic Nagyerdei krt. 98 Debrecen, Hungary H-4032
-

Monika Bessenyei
-

+36 70 451 3768
Great Ormond Street Hospital For Children
30 Guilford St London WC1N 1EH, United Kingdom
-

Helen Cross
-

020 7405 9200
Sheffield Children's Hospital
Clarkson St, Broomhall, Sheffield S10 2TH, United Kingdom
-

Archana Desurkar
-

0114 3053236
First Department of Pediatrics at Semmelweis University
Bókay u. 53, H-1083 Budapest
-

Viktor Farkas
-

0036 20 670 1789
Tbilisi State Medical University, Givi Zhvania Academic Clinic of Pediatry
33 Lubliana Str., 0159, Tbilisi, K’alak’I T’bilisi, Georgia
-

Sophia Bakhtadze
-

+995592552929
Institute of Neurology and Neuropsychology
#83/11 Vazha-Pshavela Ave Tbilisi Georgia 0186
-

David Kvernadze
-

+995551516066
Medi Club Georgia LLC
#22 A, Tashkenti St, 0160, Tbilisi, Georgia
-

Gia Melikishvili
-

+995598357909
